

Biosimilarity
2 587 kr
2 587 kr
Tis, 18 feb - fre, 21 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Produktbeskrivning
Summary:
The focus of this book is on how the U.S. FDA will approve biosimilar drugs, as learned from recent approvals by the FDA. Understanding the limitations of the statutory limits and non-inferiority testing are presented as tools to obviate patient trials and minimize testing of immunogenicity. An in-depth scientific, mathematical and statistical view of the tools required to establish biosimilarity of biological drugs of different complexity -- a must for every developer of biosimilars.
Features:
First comprehensive analysis based on new guidelines and approval packages of several biosimilars
Presents the first approach to challenge FDA in reducing or eliminating any testing in patients.
Provides a comprehensive understanding of the U.S. statutory requirements vis-a-vis the regulatory guidelines
Provides model CQA and Analytical Similarity testing protocols for cytokines and monoclonal antibodies
Allow creation of a fast-to-market pathway to develop biosimilars
Artikel.nr.
b0b49970-6d63-42f9-aa0f-83370aceacc2
Biosimilarity
2 587 kr
2 587 kr
Tis, 18 feb - fre, 21 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Liknande toppsäljare

Squid Game 2 Gonggi & Case Korean
99 kr

INF TYPE-C Dubbel SD/TF-kortläsare för snabb dataöverföring 0
89 kr
Tidigare lägsta pris:
103 kr

UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
879 kr
Tidigare lägsta pris:
939 kr

FENCHILIN Stor Hollywood sminkspegel med belysning USB Bordsskiva Väggfäste Vit spegel 80 x 58 cm
1 421 kr
Tidigare lägsta pris:
1 611 kr

Squid Game 2 Gonggi & Round Case koreanska
129 kr

Wii till HDMI-adapter, 1080p Full-HD Nintendo
67 kr

Korbell Refill 3-pack
199 kr

Justerbar Odlingslampa - LED, Timer
249 kr

RCA AV till HDMI Converter / Adapter 1080P Universal White
99 kr

Samsung Galaxy Tab A9+ Wifi 64GB Svart
2 210 kr
Tidigare lägsta pris:
2 345 kr
Rekommendationer för dig

Öronkuddar för Bose QuietComfort - QC35/QC25/QC15/AE2 Hörlurar Svart
99 kr

Vevradio med Solceller, LED Ficklampa och 2000mAh Powerbank SOS SUNMATIC - Väderradio för hem- och campingnödsituationer med AM/FM-radio
289 kr

INF Cocktail set Dubbel shaker 750ml Rostfritt stål Silver 10 delar
269 kr
Tidigare lägsta pris:
385 kr

12-pack Oral-B Kompatibla Tandborsthuvuden
79 kr
Tidigare lägsta pris:
99 kr
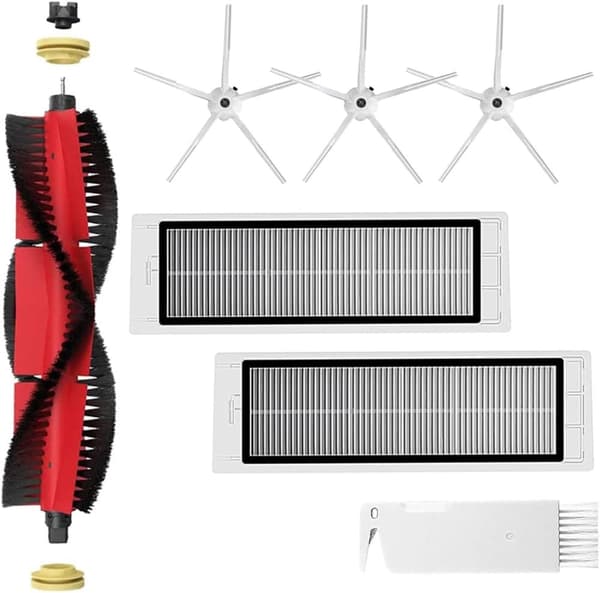
INF Tillbehör för Roborock S5/S6 modeller 7 delar
169 kr
Tidigare lägsta pris:
199 kr

PS4 Handkontroll DoubleShock Trådlös för Play-station 4
269 kr
Tidigare lägsta pris:
299 kr

Apple AirPods Pro (2nd Generation) USB-C
2 876 kr

UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
879 kr
Tidigare lägsta pris:
939 kr

Apple AirPods (andra generation) med Lightning-laddningsetui
1 494 kr

Mini trådlös CarPlay & Android Auto-adapter för iPhone & Android – Njut av friheten från sladdar
379 kr
Tidigare lägsta pris:
399 kr