
Post-Authorization Safety Studies of Medicinal Products
1 379 kr
1 379 kr
I lager
Ons, 19 feb - tis, 25 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Produktbeskrivning
Post-Authorization Safety Studies of Medicinal Products: The PASS Book bridges the gap in the literature by providing a complete look at post-authorization safety studies and important pharmacoepidemiology and pharmacovigilance aspects. It covers various types and limitations of active surveillance programs, including the use of large databases and disparate data sources for rapid signal detection, as well as novel and advanced design and analysis approaches for causal interference from observational data. This book serves as an important reference for pharmacovigilance scientists and pharmacoepidemiologists who are searching for the appropriate study design to answer safety research questions. Readers will be able to effectively and efficiently design and interpret findings from post-authorization safety studies with the goal of improving the benefit-risk balance of a drug in order to optimize patient safety.
Artikel.nr.
0fd58293-4c00-467d-b63f-c71781605d3f
Post-Authorization Safety Studies of Medicinal Products
1 379 kr
1 379 kr
I lager
Ons, 19 feb - tis, 25 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Liknande toppsäljare

Generic
Öronkuddar för Bose QuietComfort - QC35/QC25/QC15/AE2 Hörlurar Svart
99 kr
4,5(736)
onsdag, 12 feb

CherrysC
Squid Game 2 Gonggi & Round Case koreanska
129 kr
torsdag, 13 feb

Apple
Apple AirPods (andra generation) med Lightning-laddningsetui
1 448 kr
3,5(98)
tisdag, 11 feb

Macaro
12-pack Oral-B Kompatibla Tandborsthuvuden
79 kr
Tidigare lägsta pris:
99 kr
3,4(68)
måndag, 17 feb

Apple
Apple AirPods Pro (2nd Generation) USB-C
2 878 kr
3,0(2)
tisdag, 11 feb

Rivanoo
Mini trådlös CarPlay & Android Auto-adapter för iPhone & Android – Njut av friheten från sladdar
379 kr
Tidigare lägsta pris:
399 kr
4,1(8)
onsdag, 12 feb

Uniq Vanity
UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
879 kr
Tidigare lägsta pris:
939 kr
tisdag, 11 feb

CherrysC
Squid Game 2 Gonggi & Case Korean
99 kr
3,0(5)
torsdag, 13 feb

SUNMATIC
Vevradio med Solceller, LED Ficklampa och 2000mAh Powerbank SOS SUNMATIC - Väderradio för hem- och campingnödsituationer med AM/FM-radio
289 kr
4,8(5)
fredag, 14 feb

Izoxis
Nattlampa - Stjärnprojektor med LED-lampa - Astronaut
349 kr
3,9(33)
onsdag, 12 feb
Rekommendationer för dig

INF
INF TYPE-C Dubbel SD/TF-kortläsare för snabb dataöverföring 0
89 kr
Tidigare lägsta pris:
103 kr
5,0(2)
onsdag, 12 feb

A Series
RCA AV till HDMI Converter / Adapter 1080P Universal White
99 kr
4,0(9)
tisdag, 11 feb

INF
INF LED Spotlights Set – 6 stilrena lampor med 2 praktiska fjärrkontroller
179 kr
Tidigare lägsta pris:
237 kr
3,8(61)
onsdag, 12 feb

Fenchilin
FENCHILIN Stor Hollywood sminkspegel med belysning USB Bordsskiva Väggfäste Vit spegel 80 x 58 cm
1 421 kr
Tidigare lägsta pris:
1 611 kr
4,6(189)
onsdag, 12 feb

DaxBox
DP - Displayport (hane) till HDMI adapter (hona) 4K-1080P
69 kr
4,7(7)
måndag, 17 feb

Uniq Vanity
UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
879 kr
Tidigare lägsta pris:
939 kr
4,5(33)
tisdag, 11 feb

Generic
Visuell Timer för Klassrum - 60 minuter
229 kr
Tidigare lägsta pris:
349 kr
4,2(56)
onsdag, 12 feb

Megabilligt
Sovhörlurar – Pannband & Ögonmask med Bluetooth Hörlurar
179 kr
Tidigare lägsta pris:
249 kr
4,1(22)
onsdag, 12 feb
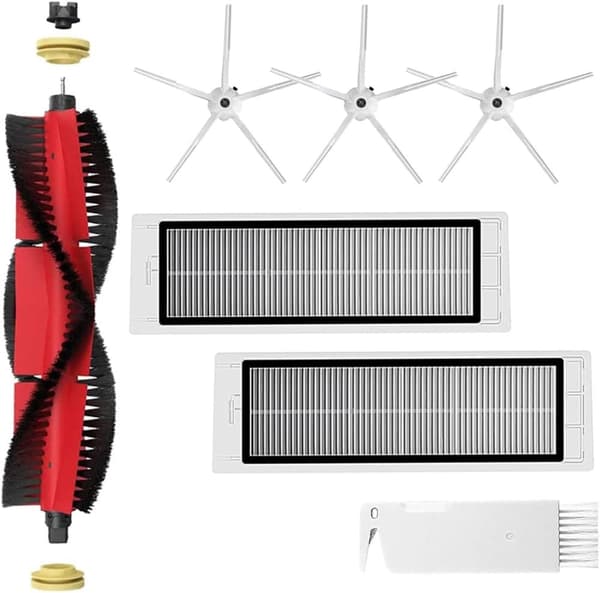
INF
INF Tillbehör för Roborock S5/S6 modeller 7 delar
169 kr
Tidigare lägsta pris:
199 kr
4,2(50)
onsdag, 12 feb

SAMSUNG
Samsung Galaxy Tab A9+ Wifi 64GB Svart
2 210 kr
Tidigare lägsta pris:
2 345 kr
4,5(80)
tisdag, 11 feb