
Regulatory Affairs in the Pharmaceutical Industry
2 142 kr
2 142 kr
I lager
Ons, 19 feb - tis, 25 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Produktbeskrivning
Regulatory Affairs in the Pharmaceutical Industry is a comprehensive reference that compiles all the information available pertaining to regulatory procedures currently followed by the pharmaceutical industry. Designed to impart advanced knowledge and skills required to learn the various concepts of regulatory affairs, the content covers new drugs, generic drugs and their development, regulatory filings in different countries, different phases of clinical trials, and the submission of regulatory documents like IND (Investigational New Drug), NDA (New Drug Application) and ANDA (Abbreviated New Drug Application). Chapters cover documentation in the pharmaceutical industry, generic drug development, code of Federal Regulation (CFR), the ANDA regulatory approval process, the process and documentation for US registration of foreign drugs, the regulation of combination products and medical devices, the CTD and ECTD formats, and much more.
Artikel.nr.
0042752c-183c-4961-8418-b6b587bdc06e
Regulatory Affairs in the Pharmaceutical Industry
2 142 kr
2 142 kr
I lager
Ons, 19 feb - tis, 25 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Liknande toppsäljare

CherrysC
Squid Game 2 Gonggi & Round Case koreanska
129 kr
torsdag, 13 feb

CherrysC
Squid Game 2 Gonggi & Case Korean
99 kr
3,3(7)
torsdag, 13 feb

Apple
Apple AirPods (andra generation) med Lightning-laddningsetui
1 494 kr
3,6(97)
tisdag, 11 feb

Macaro
12-pack Oral-B Kompatibla Tandborsthuvuden
79 kr
Tidigare lägsta pris:
99 kr
3,4(68)
måndag, 17 feb
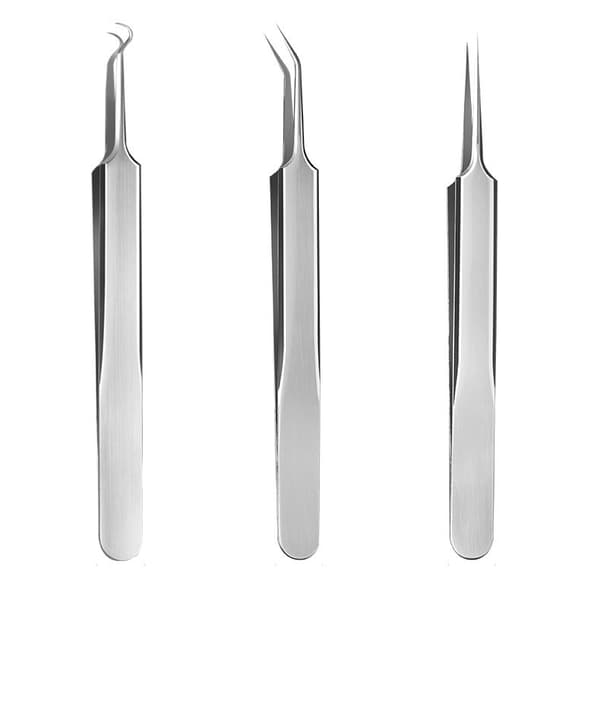
INF
Pormaskborttagare i rostfritt stål, set med 3 instrument Silver
89 kr
4,5(6)
fredag, 21 mar

Korbell
Korbell Refill 3-pack
199 kr
4,7(52)
torsdag, 13 feb

Uniq Vanity
UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
879 kr
Tidigare lägsta pris:
939 kr
tisdag, 11 feb

SAMSUNG
Samsung Galaxy Tab A9+ Wifi 64GB Svart
2 210 kr
Tidigare lägsta pris:
2 345 kr
4,5(81)
tisdag, 11 feb

Generic
Öronkuddar för Bose QuietComfort - QC35/QC25/QC15/AE2 Hörlurar Svart
99 kr
4,5(738)
onsdag, 12 feb

SUNMATIC
Vevradio med Solceller, LED Ficklampa och 2000mAh Powerbank SOS SUNMATIC - Väderradio för hem- och campingnödsituationer med AM/FM-radio
289 kr
4,8(5)
fredag, 14 feb
Rekommendationer för dig

INF
INF TYPE-C Dubbel SD/TF-kortläsare för snabb dataöverföring 0
89 kr
Tidigare lägsta pris:
103 kr
5,0(2)
onsdag, 12 feb

Nintendo Switch
Hogwarts Legacy - Nintendo Switch
387 kr
Tidigare lägsta pris:
408 kr
4,8(4)
fredag, 14 feb

A Series
RCA AV till HDMI Converter / Adapter 1080P Universal White
99 kr
4,0(9)
tisdag, 11 feb

RockJam
RockJam 61-tangenters Keyboard Piano Kit med Pall, Stativ, Hörlurar & Lektioner
1 299 kr
5,0(5)
onsdag, 12 feb

Fenchilin
FENCHILIN Stor Hollywood sminkspegel med belysning USB Bordsskiva Väggfäste Vit spegel 80 x 58 cm
1 421 kr
Tidigare lägsta pris:
1 611 kr
4,6(189)
onsdag, 12 feb

Rivanoo
Mini trådlös CarPlay & Android Auto-adapter för iPhone & Android – Njut av friheten från sladdar
379 kr
Tidigare lägsta pris:
399 kr
4,2(9)
onsdag, 12 feb

Apple
Apple AirPods Pro (2nd Generation) USB-C
2 876 kr
3,0(2)
tisdag, 11 feb

Uniq Vanity
UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
879 kr
Tidigare lägsta pris:
939 kr
4,5(34)
tisdag, 11 feb

Megabilligt
Sovhörlurar – Pannband & Ögonmask med Bluetooth Hörlurar
179 kr
Tidigare lägsta pris:
249 kr
4,1(22)
onsdag, 12 feb
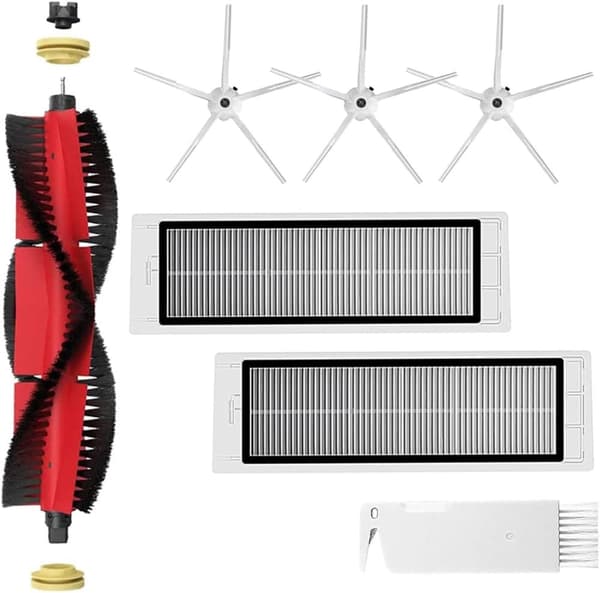
INF
INF Tillbehör för Roborock S5/S6 modeller 7 delar
169 kr
Tidigare lägsta pris:
199 kr
4,2(50)
onsdag, 12 feb