
Taylor & francis ltd
Writing In-House Medical Device Software in Compliance with EU, UK, and US Regulations (häftad, eng)
649 kr
649 kr
Fre, 7 feb - mån, 10 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Buyersclub.se
Produktbeskrivning
This book is a comprehensive guide to producing medical software for routine clinical use. It is a practical guidebook for medical professionals developing software to ensure compliance with medical device regulations for software products intended to be sold commercially, shared with healthcare colleagues in other hospitals, or simply used in-house.
It compares requirements and latest regulations in different global territories, including the most recent EU regulations as well as UK and US regulations.
This book is a valuable resource for practising clinical scientists producing medical software in-house, in addition to other medical staff writing small apps for clinical use, clinical scientist trainees, and software engineers considering a move into healthcare.
The academic level is post-graduate, as readers will require a basic knowledge of software engineering principles and practice.
Key Features:
- Up to date with the latest regulations in the UK, the EU, and the US
- Useful for those producing medical software for routine clinical use
- Contains best practice
Format Häftad Omfång 278 sidor Språk Engelska Förlag Taylor & Francis Ltd Utgivningsdatum 2024-03-26 ISBN 9781032293509
Artikel.nr.
18cc1231-8dd1-598f-9597-91a0a3f1db69
Taylor & francis ltd
Writing In-House Medical Device Software in Compliance with EU, UK, and US Regulations (häftad, eng)
649 kr
649 kr
Fre, 7 feb - mån, 10 feb
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Buyersclub.se
Liknande toppsäljare

Squid Game 2 Gonggi & Case Korean
99 kr

12-pack Oral-B Kompatibla Tandborsthuvuden
89 kr

UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
879 kr
Tidigare lägsta pris:
939 kr

Squid Game 2 Gonggi & Round Case koreanska
129 kr

Sony PlayStation DualSense - White (PS5)
779 kr

Löpband under skrivbordet 1-6KM/H Walking Jogging Machine för hemmakontor Svart
1 849 kr

Vevradio med Solceller, LED Ficklampa och 2000mAh Powerbank SOS SUNMATIC - Väderradio för hem- och campingnödsituationer med AM/FM-radio
289 kr
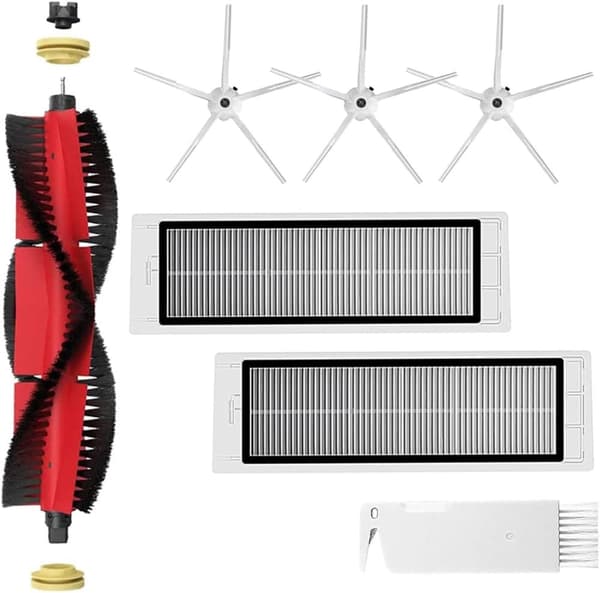
INF Tillbehör för Roborock S5/S6 modeller 7 delar
169 kr
Tidigare lägsta pris:
199 kr

Apple AirPods (andra generation) med Lightning-laddningsetui
1 448 kr

PlayStation 5 Slim Standard Edition (PS5)
6 490 kr
Tidigare lägsta pris:
6 899 kr
Rekommendationer för dig

iPhone Laddare Snabbladdare - Adapter + Kabel 20W USB-C 2m
99 kr
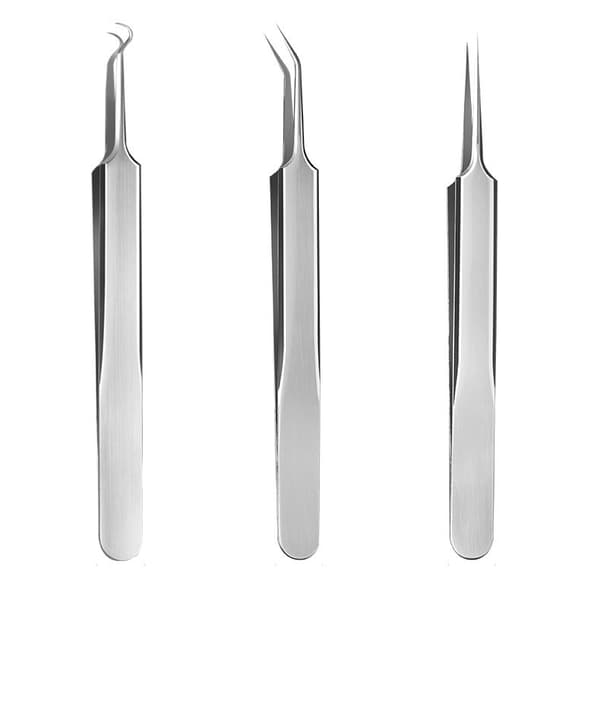
Pormaskborttagare i rostfritt stål, set med 3 instrument Silver
89 kr

Laddare för iPhone 15 / iPhone 16 + 2M kabel Snabbladdare USB-C till USB-C
99 kr

FENCHILIN Stor Hollywood sminkspegel med belysning USB Bordsskiva Väggfäste Vit spegel 80 x 58 cm
1 421 kr
Tidigare lägsta pris:
1 611 kr

12-pack Oral-B Kompatibla Tandborsthuvuden
79 kr
Tidigare lägsta pris:
99 kr

Nattlampa - Stjärnprojektor med LED-lampa - Astronaut
349 kr

2-Pack - Laddare för iPhone Adapter+Kabel 20W USB-C Snabbladdare
199 kr

Öronkuddar för Bose QuietComfort - QC35/QC25/QC15/AE2 Hörlurar Svart
99 kr

RCA AV till HDMI Converter / Adapter 1080P Universal White
99 kr

INF Cocktail set Dubbel shaker 750ml Rostfritt stål Silver 10 delar
269 kr
Tidigare lägsta pris:
385 kr