
Specification of Drug Substances and Products
2 168 kr
2 168 kr
Tidigare lägsta pris:
2 252 kr
Fre, 30 maj - tor, 5 jun
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Produktbeskrivning
Artikel.nr.
9f3ae594-b3db-4b83-b91f-4a92079298af
Specification of Drug Substances and Products
2 168 kr
2 168 kr
Tidigare lägsta pris:
2 252 kr
Fre, 30 maj - tor, 5 jun
Säker betalning
14-dagars öppet köp
Säljs och levereras av
Adlibris
Liknande toppsäljare

Apple AirPods 4 Wireless In-ear
1 559 kr
Tidigare lägsta pris:
1 569 kr

Apple AirPods Pro (andra generationen) 2023 med MagSafe-fodral (USB-C)
2 549 kr

Elastiskt träningsband till fotboll - Gul/Svart
114 kr

3-pack ersättningsblad för Philips OneBlade för män
199 kr
Tidigare lägsta pris:
209 kr

Kompatibel iPhone snabbladdare USB-C strömadapter 20W + 2m Kabel
99 kr
Tidigare lägsta pris:
119 kr

Röd Vevradio med Solceller, Ficklampa och 2000mAh Powerbank Nödradio, Överlevnad
149 kr
Tidigare lägsta pris:
436 kr

Laddare för iPhone 15 / iPhone 16 + 2M kabel Snabbladdare USB-C till USB-C
99 kr

FENCHILIN Hollywood sminkspegel med belysning 360° vridbar spegel med förstoring dimbar med tre ljuslägen bordsskiva spegel med lampor Vit 65 x 49 cm
795 kr
Tidigare lägsta pris:
933 kr

Sony PlayStation DualSense - White (PS5)
798 kr
Tidigare lägsta pris:
847 kr

POP MART Labubu The Monsters Macaron 17 cm
159 kr
Rekommendationer för dig

Munspel - C-durskala
99 kr

INF TYPE-C Dubbel SD/TF-kortläsare för snabb dataöverföring 0
79 kr

Fotbollsmål i Metall med Prickskytteduk - 240x170cm
999 kr
Tidigare lägsta pris:
1 299 kr
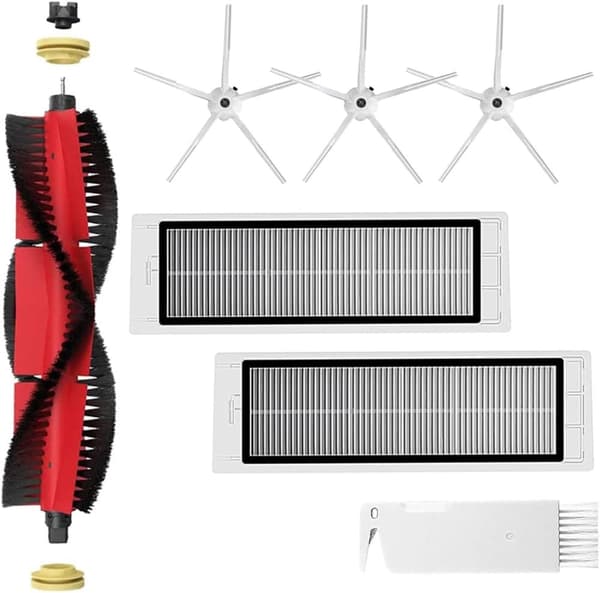
INF Tillbehör för Roborock S5/S6 modeller 7 delar
149 kr
Tidigare lägsta pris:
199 kr
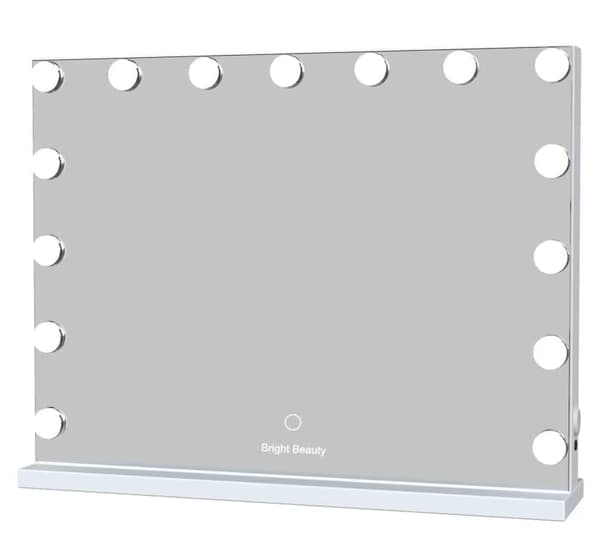
Bright Beauty Vanity Namira - sminkspegel med belysning - hollywoodspegel - make up spegel - vit - dimbar med tre ljuslägen
849 kr
Tidigare lägsta pris:
899 kr

Laddare till Samsung 25W - Snabbladdare USB-C - Strömadapter+Kabel 1M
99 kr
Tidigare lägsta pris:
119 kr

INF 6-pack trimmerspolar med trimmertråd och lock RYOBI grästrimmer
142 kr
Tidigare lägsta pris:
189 kr

Pool Robot Scooby
4 999 kr
Tidigare lägsta pris:
5 429 kr

Hopfällbar Trädgårdspall med Verktyg / Knäpall - Pall
349 kr
Tidigare lägsta pris:
399 kr

Vattenfontän solcellsdriven 16 cm solcellsfontän vatten fontän utomhus Svart
179 kr