
Safety Risk Management for Medical Devices
1 521 kr
1 521 kr
Ons, 18 jun - ons, 25 jun
Säker betalning
14-dagars öppet köp
Säljs och levereras av
AdlibrisProduktbeskrivning
Artikel.nr.
be6a3d2e-6760-4360-82b3-beb04aef8b3b
Safety Risk Management for Medical Devices
1 521 kr
1 521 kr
Ons, 18 jun - ons, 25 jun
Säker betalning
14-dagars öppet köp
Säljs och levereras av
AdlibrisLiknande toppsäljare

POP MART Labubu The Monsters – Exciting Macaron Blind Box 17 cm Vinylfigur | Samlarobjekt | Designer Toy | Originalprodukt
179 kr

1 st POP MART Labubu The Monsters Exciting Macaron Plyschfigur Leksak Blind Box (slumpad färg, 17 cm, 1 pack)
179 kr

1 st POP MART Labubu 2.0 The Monsters Macaron Blind Box Plyschfigur (slumpad färg, 17 cm, generation 2, 1-pack)
199 kr
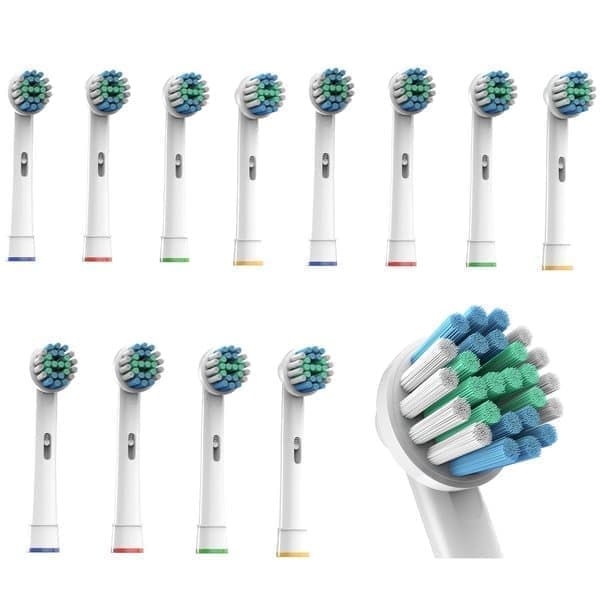
12-pack Oral-B Kompatibla Tandborsthuvuden
89 kr

POP MART Labubu The Monsters Big into Energy 17 cm
199 kr

Vattenfontän solcellsdriven 16 cm solcellsfontän vatten fontän utomhus Svart
149 kr
Tidigare lägsta pris:
179 kr

1 st POP MART Labubu 3.0 Big into Energy Blind Box Figur – The Monsters Vinyl Plysch Hänge 17 cm (slumpad färg, enkel pack)
199 kr

Sony PlayStation DualSense - White (PS5)
639 kr
Tidigare lägsta pris:
679 kr

Samsung Galaxy Buds3 Pro - Silver
1 598 kr
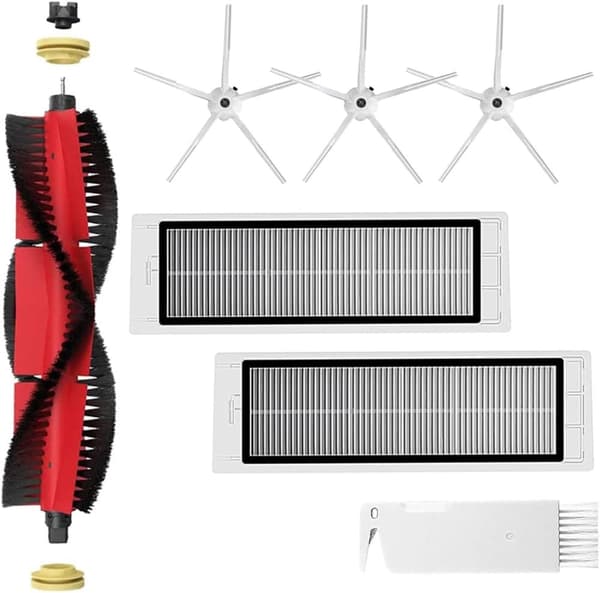
INF Tillbehör för Roborock S5/S6 modeller 7 delar
149 kr
Tidigare lägsta pris:
199 kr
Rekommendationer för dig

INF TYPE-C Dubbel SD/TF-kortläsare för snabb dataöverföring 0
79 kr

Apple AirPods 4 Active Noise Cancellation Wireless In-ear
2 096 kr
Tidigare lägsta pris:
2 099 kr

Samsung Galaxy Tab A9+ Wifi 64GB Svart grafit
1 980 kr

Playstation 5 DualSense Controller Midnight Black
639 kr
Tidigare lägsta pris:
679 kr

iPhone Snabbladdare USB-C PD 3.0. 20W Strömadapter + Kabel
117 kr

Apple Iphone 16e 128GB Svart Svart
7 390 kr

UNIQ XL Hollywood Spegel med 15 LED-lampor och touch-funktion - sminkspegel med belysning - hollywoodspegel
795 kr
Tidigare lägsta pris:
895 kr

Laddare för iPhone 15 / iPhone 16 + 2M kabel Snabbladdare USB-C till USB-C
99 kr

PlayStation 5 Slim Digital Edition (PS5)
5 219 kr
Tidigare lägsta pris:
6 589 kr

INF Cocktail set Dubbel shaker 750ml Rostfritt stål Silver 10 delar
289 kr
Tidigare lägsta pris:
385 kr